In situ measurements of nighttime polar (negative and positive) ion
conductivity were performed by Holzworth et al. (1985). The polar
conductivity values were found to be nearly equal
(
![]() ) at most altitudes.
Holzworth et al. (1985) measured ion conductivities which were well
fit by an exponential scale height of 8.0 km below 40 km altitude and
11.1 km between 40 and 56 km altitude. The total ion conductivity
(
) at most altitudes.
Holzworth et al. (1985) measured ion conductivities which were well
fit by an exponential scale height of 8.0 km below 40 km altitude and
11.1 km between 40 and 56 km altitude. The total ion conductivity
(
![]() ) at 40 km was measured to
be .
) at 40 km was measured to
be .
Holzworth et al. (1985) found a significant departure from the
11.1 km scale height above ![]() 56 km altitude with a significant
drop in
56 km altitude with a significant
drop in ![]() measured at 65 km altitude. The
measured at 65 km altitude. The ![]() profile above 56 km will be approximated by an exponential with the
scale height determined from the measured
profile above 56 km will be approximated by an exponential with the
scale height determined from the measured ![]() values at or
near the endpoints of 56 km and 72 km. The total ion conductivity at
56 km using Equation 2.16 is
1.69
values at or
near the endpoints of 56 km and 72 km. The total ion conductivity at
56 km using Equation 2.16 is
1.69![]() 10
10![]() S/m while that at 70 km was measured by
Holzworth et al. (1985) to be
S/m while that at 70 km was measured by
Holzworth et al. (1985) to be ![]() 4.0
4.0![]() 10
10![]() S/m. The
exponential scale height based on these points would be about 16.3 km.
The total ion conductivity profile is summarized in the equations
below.
S/m. The
exponential scale height based on these points would be about 16.3 km.
The total ion conductivity profile is summarized in the equations
below.
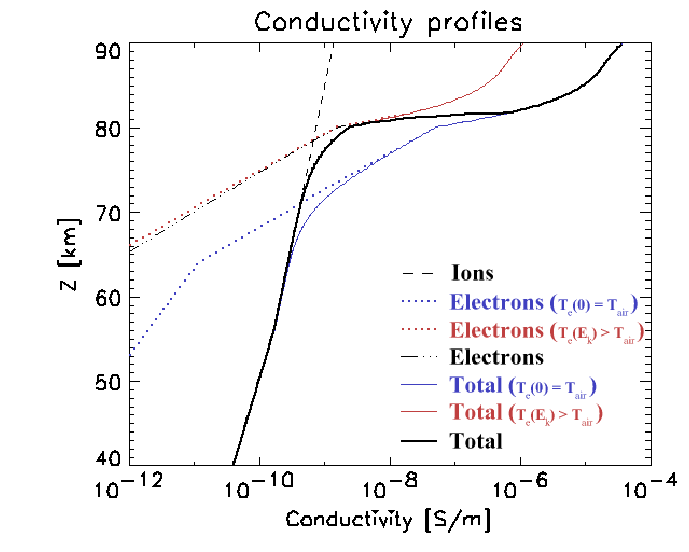
The dashed line in Figure 2.5 shows the ionic
conductivity, ![]() , for 40-90 km altitude, based on
Equations 2.15-2.17.
Equation 2.17 was extended to altitudes above
72 km, even though Holzworth et al. (1985) did not obtain any
measurements in that region. This extrapolation may be somewhat in
error for altitudes of
, for 40-90 km altitude, based on
Equations 2.15-2.17.
Equation 2.17 was extended to altitudes above
72 km, even though Holzworth et al. (1985) did not obtain any
measurements in that region. This extrapolation may be somewhat in
error for altitudes of ![]() 72-78 km. However, the increasing
importance of the electron conductivity with increasing altitude will
diminish the potential impact of such errors (see next section).
72-78 km. However, the increasing
importance of the electron conductivity with increasing altitude will
diminish the potential impact of such errors (see next section).
 |